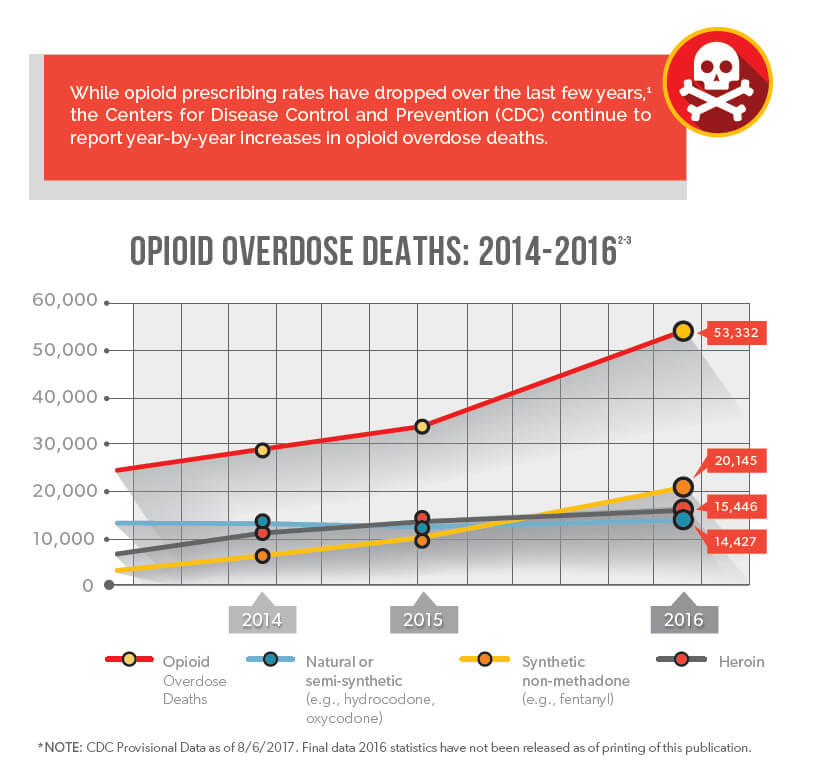
Various industry stakeholders have implemented a multitude of practices, guidelines, and regulations in order to combat the opioid epidemic at every possible opportunity, and they have achieved many significant victories. However, opioid overdose deaths have continued to increase, most recently due to a significant rise in the use of synthetic opioids such as fentanyl. As the opioid landscape continues to evolve, there is still a strong need for continued opioid policy change.
As the federal agency responsible for protecting and promoting public health through the control and supervision of pharmaceuticals, the U.S. Food and Drug Administration (FDA) has recently acknowledged that there is a significant gap in their understanding of how the opioid products they approve are used in the real world.
The FDA has therefore announced a variety of initiatives intended to tighten opioid policy in the interest of public safety. This includes a reevaluation of the effectiveness of abuse-deterrent opioid formulations, influencing prescriber practices with expanded rules and education, reassessing organizational decision-making strategies, and more.
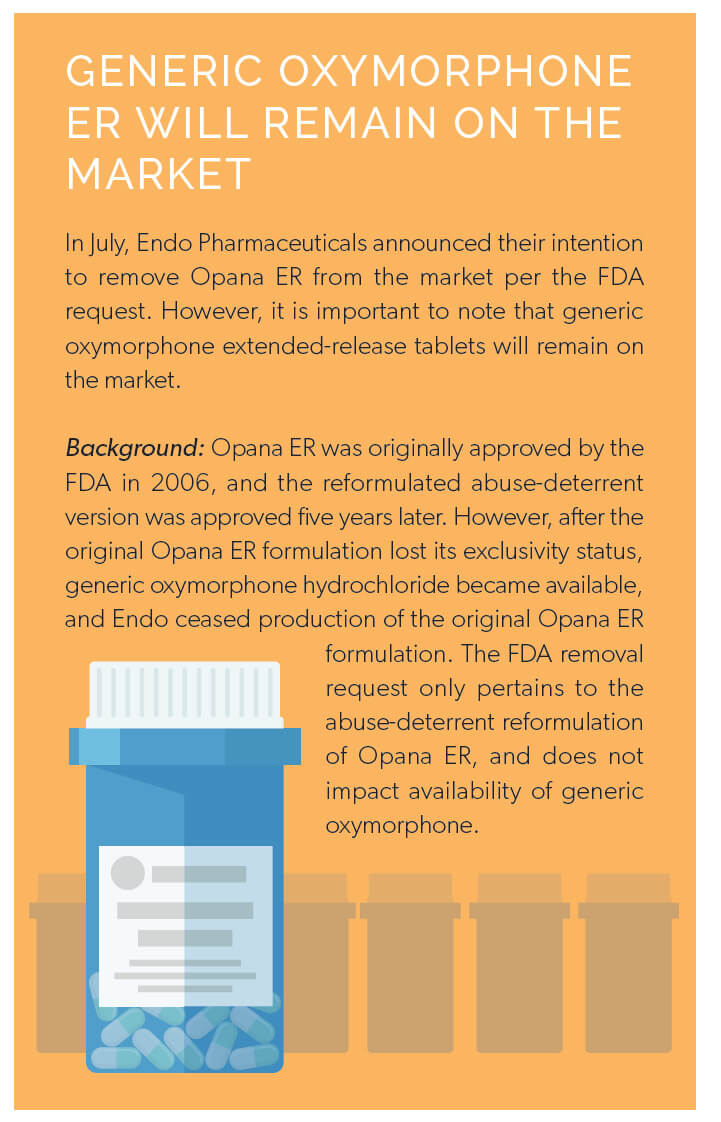
In June, the FDA stunned healthcare professionals by asking Endo Pharmaceuticals to remove the abuse-deterrent formulation of Opana ER from the market. The FDA request came after post-market data tied the drug to a surge in injection-based abuse that led to serious outbreaks of HIV and hepatitis C virus (HCV).4 This was the first time the FDA requested the removal of a currently marketed opioid pain medication from the market due to public health concerns. Less than a week later, the FDA announced a public meeting to discuss with external thought leaders whether or not abuse-deterrent opioids were having their intended impact on limiting abuse and misuse to curb the opioid epidemic.5
Within the announcement, the FDA conceded that such formulations are not abuse-proof or addiction proof, and that the organization requires a stronger understanding of how such products are used in the real world. Shortly after, the FDA announced a new study to better understand prescriber beliefs and attitudes surrounding abuse-deterrent opioids, as the FDA does not wish to improperly convey that such products are less prone to addiction.6
Over the last several years, the FDA has encouraged the development of abuse-deterrent opioids as the organization felt such formulations could offer pain relief with a reduced risk profile. However, these new initiatives acknowledge a serious disconnect between their opioid policies and the impact such policies have – or do not have – in the real world.
In order to correct such discrepancies between their policies and real-world opioid and use, the FDA is currently investigating how to better leverage existing data sources and methods to inform the regulation of abuse-deterrent opioid products.5

In an attempt to reduce overall exposure to opioids and promote appropriate opioid prescribing practices, the FDA is expanding the Risk Evaluation and Mitigation Strategy (REMS) program to immediate-release (IR) opioid products.6
REMS enforces significant prescriber education requirements in order to reduce adverse outcomes resulting from the inappropriate prescribing, misuse, and abuse of extended-release or long-acting (ER/LA) opioids. But as the FDA estimates that 90% of opioid prescriptions are for IR opioids,6 they believe REMS could help manage a larger realm of opioid prescribing. While the new REMS will differ from the original, it is worth understanding the current REMS program.
As part of REMS, all ER/LA opioid manufacturers must provide education for the prescribers of these medications, which must be provided through accredited continuing education (CE) activities. CE activities must focus on safe prescribing with a core content of three hours, covering subjects including but not limited to:
The FDA has sent detailed letters to opioid manufacturers informing them of new REMS training requirements for prescribers of IR opioids, while also instructing manufacturers that other healthcare providers, such as nurses and pharmacists, should have training made available to them as well.6
The FDA also has stated its intention to reevaluate how they make decisions in approving new drugs, how they to continue to assess drug safety for approved drugs, how to ensure general opioid prescribing is more closely tailored to the medical indication, when to revise labeling, and when to pull drugs from market.6
To date, the FDA has provided very few details on how they plan to accomplish this, but as an initial step they have opened a public commentary period to understand what areas of focus related to opioid products and policy issues are important to the public, hoping to gather feedback from a variety of industry professionals. As of the publication of this article, the FDA plans to accept commentary until December 28, 2017, seeking input as it considers how its authorities can or should be used to address this crisis.8
The announcement of such goals implies a potential plethora of changes aimed at curbing the opioid epidemic. However, the collective size and scope of such changes could be an obstacle in and of itself. Restructuring such wide-impacting policies would demand significant time, communication, and resources. As with most major federal undertakings, the road ahead will likely take many turns, possibly resulting in outcomes different than originally planned, but this arduous undertaking is incredibly necessary to reduce the harm of an epidemic that has raged for over a decade.
In March of 2016, the FDA formally asked the National Academies of Sciences, Engineering and Medicine (NASEM) to outline the state of the science regarding prescription opioid abuse and misuse.9 NASEM produced an extensive report, making various recommendations to the FDA, who have followed many of those recommendations either deliberately or coincidentally while reassessing their policies internally.
However, many of NASEM’s recommendations have not yet been addressed, and it is not clear if the FDA has rejected such recommendations or if they are still considering them. It is therefore possible that some of these recommendations could be taken in the near future, including:10
Because it remains to be seen just how the FDA will carry out these new initiatives, it is unknown what impacts these initiatives will have on opioid use. However, it is worth noting that this course of action is a significant departure from previous FDA oversight, and it could be a welcome departure if these initiatives encourage further movement away from opioid prescribing.
As a significant authority on drug policy, the FDA has the power to reshape opioid management by taking stronger regulatory ownership. The implementation of stronger opioid policies could give healthcare professionals some additional framework for protecting patient safety. We will continue to monitor the FDA’s actions and any resulting impacts on this epidemic.