An estimated 100 million Americans suffer from chronic pain1, and as the devastating impacts of the opioid epidemic continue to reverberate, there remains a significant and unmet need for non-opioid pain medications that can safely and effectively
manage pain, without presenting the risks posed by opioids.
One trending model drug developers are employing to meet this need is the creation of new medications that reduce pain by targeting different neurochemcial pain pathways in the brain, going beyond traditional opioid pathways.
Opioid medications produce their analgesic effects by binding to special opioid receptors in the brain, triggering nerve cells to reduce pain messages sent to the brain. However, stimulating these opioid receptors is also what leads to adverse drug effects, such as respiratory depression, nausea, and importantly, the euphoric high that can eventually contribute to dependence, misuse, and abuse.
Despite the risks involved, opioids have remained popular because this mechanism of action is incredibly effective in providing pain relief. But as science continues to progress, researchers have increasingly been targeting other neuropathways in the brain that play active roles in the transmission of pain.
The FDA recently granted Fast Track designation to tanezumab, a non-opioid biologic currently in Phase 3 development for the treatment of chronic low-back pain and osteoarthritis.2 Tanezumab is a special antibody that targets, binds to, and inhibits nerve growth factor (NGF). NGF is an important mediator of pain initiation and maintenance, and researchers believe pharmacotherapies that target this pathway could show significant promise in the treatment of pain.3 Clinical trials for tanezumab are expected to produce results in 2018, and tanezumab’s Fast Track designation increases collaboration between drug developers and the FDA, which could improve the drug’s chances of FDA approval as the FDA would offer more insight and feedback.
To qualify for Fast Track status, drugs must treat unmet medical needs. The FDA’s decision to Fast Track tanezumab indicates the agency’s belief that drugs like tanezumab, which target non-opioid receptors involved in the sensation of pain, have the potential to effectively treat pain with fewer risks than currently available opioid medications.
This potentially opens the door to more investment in drugs that target non-opioid pain neuropathways, which could lead to a wave of new pain medications.
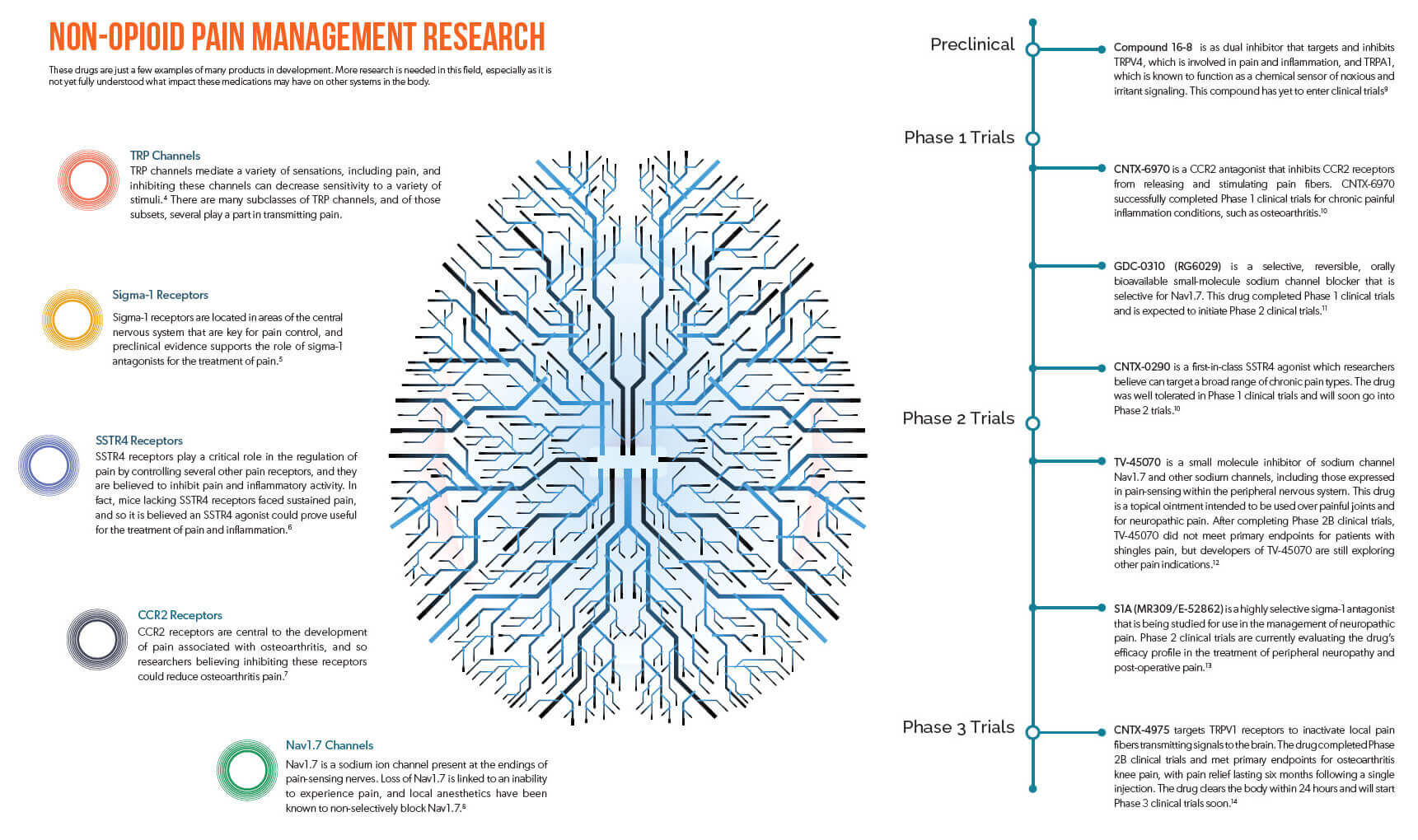
The field of neuroscience is incredibly complex, but researchers have been studying a wide range of other neuropathways that help transmit pain. In some cases, they have developed compounds that can affect those neuropathways to impact pain, and an exploration of these alternatives may serve to benefit patients in the near future, if these products prove safe and effective. (View the infographic below for an overview of current research being undertaken in neuropathways, and associated molecules in development.)
Novel, non-opioid pain medications could potentially offer patients fewer risks with significant improvements in quality of life. However, it must be acknowledged that with new pathways come new challenges. These novel medications may come with their own adverse effects, and the interconnected nature of neuropathways could lead to such drugs significantly impacting other systems within the body.
As novel products such as tanezumab eventually become available, clinical evidence will ultimately guide prescribing as it relates to pain and workplace injury. If these novel agents disrupt the pain therapy landscape in any significant way, the industry may see treatment guidelines shift to consider their role in treatment. Furthermore, the introduction of such new, high-cost specialty drugs could increase spending. It is therefore imperative that we remain vigilant for further drug developments as they have the potential to change our industry.